Buy Sodium Bisulfite (7631-90-5) MF: NaHSO3 | MW: 104.06. Get High Quality Sodium Bisulfite (7631-90-5) from ChemieR.
Sodium bisulfite ACS grade is a white, crystalline solid with a sulfurous odor. It is soluble in water and forms a mildly acidic solution. It is commonly prepared by bubbling sulfur dioxide gas through a solution of sodium carbonate. Sodium bisulfite is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite in fact is not a real compound,but a mixture of salts that dissolve in water to give solutions comIt contains a hydrogensulfite. Its synonyms are Sulfurous Acid Monosodium Salt ; Sodium Hydrogen Sulfite. Sodium bisulfite is a widely used industrial chemical as a reducing agent, catalyst, and preservative in pharmaceuticals and foods due to its antioxidative and antibacterial effects. The aqueous solutions of sodium bisulfite are acidic in nature. Degradation of sodium bisulfite with acid forms sulfur dioxide gas. ACS grade sodium bisulfite ensures high purity, which is essential for accurate laboratory analysis and experimentation. Sodium bisulfite ACS grade is handled with proper safety precautions due to its corrosive and irritant properties, but when used appropriately, it poses minimal risks.
ChemieR offers a variety of high purity salts and solutions of alkali metals, alkaline earth compounds, perchlorates, heavy metals, rare earths, transition metals, silver/precious metals, and triflates. ChemieR covers wide range of inorganic chemicals, specializing in low moisture and low trace metal grade materials.
ChemieR is innovative and unique product range in laboratory chemicals which includes organic reagents, inorganic reagents, and solvents. Order from our ChemieR brand to meet your budget whilst not compromising on the quality.
In-addition ChemieR also distributes in numerous Laboratory Supplies, Chemicals, Equipment, Instruments, Reagents, Standard Solutions, Buffers, Biological Stains & Indicators and many more, for more information please browse our website (https://chemiereagents.com) or email us sales@chemiereagents.com we will be happy to help you. All ChemieR Products are exclusively distributed by Dawn Scientific Inc (https://dawnscientific.com)
Application :
- Sodium bisulfite (NaHSO3) has been used as a reagent to compose the immunoprecipitation (IP) buffer to chemically modify DNA and to synthesize arsenolipids (AsL).
- Sodium bisulfite can also be used as a reagent during the synthesis of 5,6-dihydrouracil-6-sulfonate.
- Sodium bisulfite can be used as a reagent to synthesize aldehyde-bisulfite adducts from aromatic and aliphatic aldehydes.
- It can also be used as a catalyst in the solvent-free etherification of aromatic/aliphatic alcohols to synthesize symmetric and unsymmetric ethers via intermolecular dehydration.
- Sodium bisulfite can also be used as an additive in the copper-catalyzed homocoupling of ketoxime carboxylates to synthesize symmetrical pyrroles.
Benefits :
- Meet the toughest regulatory standards for quality and purity
- Effective preservative
- Gives consistent and reliable results in various applications







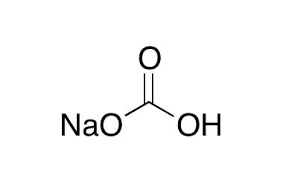

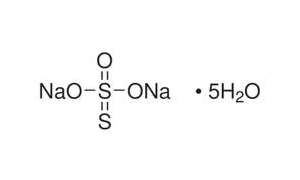

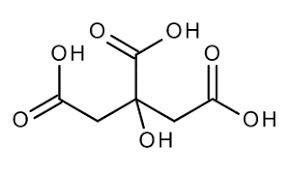

Reviews
There are no reviews yet.