Buy Sodium Acetate Anhydrous ACS (127-09-3) MF: CH3COONa • 3H2O | MW: 136.08. Get High Quality Sodium Acetate Anhydrous ACS (127-09-3) from ChemieR.
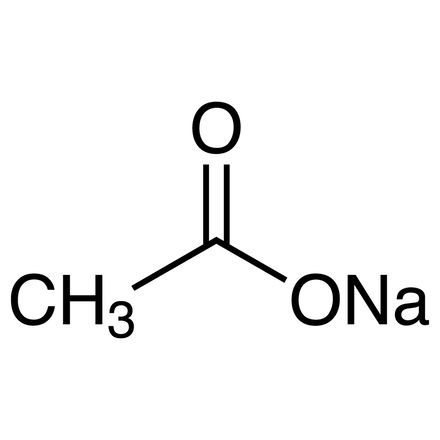
Sodium acetate anhydrous ACS typically appears as a white crystalline powder or granules. Sodium acetate anhydrous ACS is the anhydrous (water-free) form of sodium acetate. Its chemical formula is CH3COONa. Sodium acetate anhydrous ACS is soluble in water and slightly soluble in alcohol. It is also known as sodium acetate acetic acid, sodium salt and sodium ethanoate. Sodium acetate is a hydrophilic organic compound that can be prepared by treating acetic acid with sodium carbonate or sodium hydroxide. It is commonly available in ACS (American Chemical Society) grade, which signifies high purity suitable for laboratory and analytical applications. Overall, sodium acetate anhydrous ACS offers reliability, purity, and versatility, making it a valuable chemical compound in laboratory research, industrial processes, and other applications where high-quality standards are essential.
ChemieR offers a variety of high purity salts and solutions of alkali metals, alkaline earth compounds, perchlorates, heavy metals, rare earths, transition metals, silver/precious metals, and triflates. ChemieR covers wide range of inorganic chemicals, specializing in low moisture and low trace metal grade materials.
ChemieR is innovative and unique product range in laboratory chemicals which includes organic reagents, inorganic reagents, and solvents. Order from our ChemieR brand to meet your budget whilst not compromising on the quality.
In-addition ChemieR also distributes in numerous Laboratory Supplies, Chemicals, Equipment, Instruments, Reagents, Standard Solutions, Buffers, Biological Stains & Indicators and many more, for more information please browse our website (https://chemiereagents.com) or email us sales@chemiereagents.com we will be happy to help you. All ChemieR Products are exclusively distributed by Dawn Scientific Inc (https://dawnscientific.com)
Application :
- Sodium acetate anhydrous ACS can be used as a base to synthesize Oxazolones via Erlenmeyer Plochl condensation reaction of aromatic aldehydes and hippuric acid. Cinnamic acids via Perkin reaction between aromatic aldehydes and acid anhydrides.
- Sodium acetate anhydrous ACS is usually used as buffering agent with acetic acid. A mixture of sodium acetate-acetic acid buffer can be employed in the polybromination of substituted imidazoles to synthesize polybrominated imidazoles.
- It can also be used as a catalyst in tandem Knoevenagel-Michael multicomponent reactions.
- Sodium acetate anhydrous ACS is used in many areas like cosmetics, pharmaceuticals, agriculture, bronzing and textile industry.
- It is employed in production of dye materials, as a polymerization catalyst, as a polymer stabilizer, preparation of gel stains, preservative in food production and flavor enhancer in the nutrient industry.
- Sodium acetate is used in the study of lithography, photography and it is also a plating agent.
Benefits :
- Meet the toughest regulatory standards for quality and purity
- Versatile product and easy to use
- Helps to maintain stable pH when used as a buffer
- Safe and Effective






Reviews
There are no reviews yet.