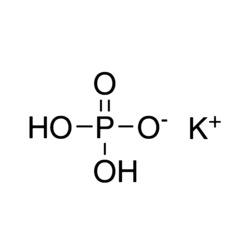
Buy Potassium Phosphate Monobasic ACS (7778-77-0) MF: KH2PO4 | MW: 136.09. Get High Quality Potassium Phosphate Monobasic ACS (7778-77-0) from ChemieR.
Potassium phosphate monobasic ACS, denoted by KH2PO4, belongs to the potassium phosphates family. As a water-soluble inorganic salt, it’s known by various names, including Monopotassium phosphate and Potassium dihydrogen phosphate. With a standard enthalpy of formation at -376.1 kcal/mol, it meets rigorous specifications set by the American Chemical Society (ACS), ensuring high purity and quality. Widely utilized in laboratories and industries for precise applications, it serves as a valuable laboratory reagent for analytical and research purposes. Specific industries may require tailored purification or adherence to different standards, like food-grade or pharmaceutical-grade specifications.
ChemieR offers a variety of high purity salts and solutions of alkali metals, alkaline earth compounds, perchlorates, heavy metals, rare earths, transition metals, silver/precious metals, and triflates. ChemieR covers wide range of inorganic chemicals, specializing in low moisture and low trace metal grade materials.
ChemieR is innovative and unique product range in laboratory chemicals which includes organic reagents, inorganic reagents, and solvents. Order from our ChemieR brand to meet your budget whilst not compromising on the quality.
In-addition ChemieR also distributes in numerous Laboratory Supplies, Chemicals, Equipment, Instruments, Reagents, Standard Solutions, Buffers, Biological Stains & Indicators and many more, for more information please browse our website (https://chemiereagents.com) or email us sales@chemiereagents.com we will be happy to help you. All ChemieR Products are exclusively distributed by Dawn Scientific Inc (https://dawnscientific.com)
Application :
- Potassium phosphate monobasic ACS acts as a starting material in the synthesis of potassium metaphosphate.
- Potassium phosphate monobasic ACS has been used in the preparation of phosphate buffer and phosphate buffered saline (PBS).
Benefits :
- Meet the toughest regulatory standards for quality and purity
- Used as a laboratory reagent or buffer
- Gives accurate and consistent results
- Versatile compound used in many industrial applications






Reviews
There are no reviews yet.