Potassium Chloride FCC/USP Grade CAS: 7447-40-7 | M.F: KCl | MW: 74.55, Buy now for Reproducible and reliable results from ChemieR Inorganic Reagent. All ChemieR Products are exclusively distributed by Dawn Scientific Inc.
Potassium Chloride FCC/USP is a substance that can be found in the form of white or colorless crystals, granules, or powder. It has a strong saline taste and its chemical formula is KCl. This compound is odorless, remains stable in air, and is highly soluble in water. Potassium chloride is classified as a metal halide salt, composed of potassium and chlorine. It is produced in large quantities by extracting it from potash ores and salt-containing surface waters. Another method of obtaining potassium chloride is through a neutralization reaction between potassium hydroxide and HCl. Approximately 5% of its global production is used as an industrial chemical, primarily for the manufacturing of potassium hydroxide and chlorine through electrolysis. Potassium Chloride USP/FCC, also known as KCL or K-Chloride, is a purified grade of potassium chloride. This grade adheres to specific purity and quality standards suitable for pharmaceutical labs and food related applications, as defined by the United States Pharmacopeia/Food Chemicals Codex.
ChemieR provides an extensive selection of high-purity salts, acids, base and high purity solvents, including alkali metals, alkaline earth compounds, perchlorates, heavy metals, rare earths, transition metals, silver and precious metals, and triflates. ChemieR specialize in inorganic chemicals, offering materials with low moisture content and minimal trace metals. ChemieR stands out with a diverse range of laboratory chemicals, from organic and inorganic reagents to solvents.
Application :
- Potassium Chloride, Crystal, FCC is used as a flavor enhancer, flavoring agent, nutrient supplement, pH control agent, and stabilizer or thickener.
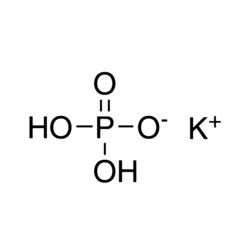
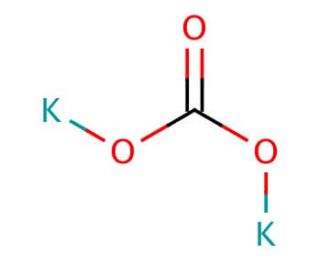
- It may be used in the preparation of potassium hydroxide, potassium carbonate and other potassium slats.
- It may be used in the calorimetric determination of enthalpy and heat capacity values during the deprotonation (loss of proton H+) of buffers. From these values temperature dependence of the pH of these buffers was calculated.
Benefits :
- Meet the toughest regulatory standards for quality and purity
- Gives reliable and consistent results
- Used as a water softner




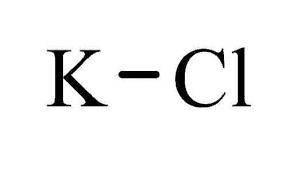








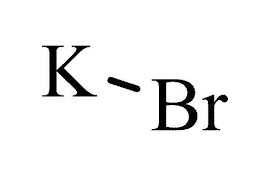

Reviews
There are no reviews yet.