Buy Potassium Carbonate Anhydrous ACS (584-08-7) MF: K2CO3 | MW: 138.21. Get High Quality Potassium Carbonate Anhydrous ACS (584-08-7) from ChemieR.
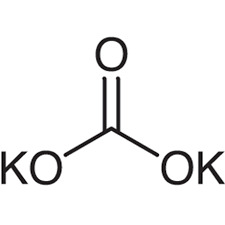
Potassium carbonate anhydrous, with the ACS (American Chemical Society) designation, is a white, granular powder that is highly soluble in water. Potassium carbonate anhydrous is an inorganic base. The chemical formula of Potassium carbonate anhydrous is K₂CO₃. It is often referred to as potash or pearl ash. It is the anhydrous form of potassium carbonate, meaning it does not contain any water molecules in its crystal structure. The ACS designation signifies that it meets the quality standards set by the American Chemical Society for laboratory use. The ACS (American Chemical Society) grade denotes a high level of purity suitable for laboratory and analytical applications. Potassium Carbonate ACS is widely used in many industrial applications like pharmaceuticals and food production. When handling any chemical substance, it’s important to follow safety guidelines and use proper laboratory practices. Additionally, specific applications and benefits may vary depending on the context and industry.
ChemieR offers a variety of high purity salts and solutions of alkali metals, alkaline earth compounds, perchlorates, heavy metals, rare earths, transition metals, silver/precious metals, and triflates. ChemieR covers wide range of inorganic chemicals, specializing in low moisture and low trace metal grade materials.
ChemieR is innovative and unique product range in laboratory chemicals which includes organic reagents, inorganic reagents, and solvents. Order from our ChemieR brand to meet your budget whilst not compromising on the quality.
In-addition ChemieR also distributes in numerous Laboratory Supplies, Chemicals, Equipment, Instruments, Reagents, Standard Solutions, Buffers, Biological Stains & Indicators and many more, for more information please browse our website (https://chemiereagents.com) or email us sales@chemiereagents.com we will be happy to help you. All ChemieR Products are exclusively distributed by Dawn Scientific Inc (https://dawnscientific.com)
Application :
- Potassium carbonate anhydrous ACS participates as a base in the palladacycle catalyzed Heck reaction of chlorobenzene with styrene.Addition of amino acids (glycine, sarcosine and proline) to K2CO3 (solvent) promotes the absorption of CO2.
- Potassium carbonate anhydrous ACS has been used in the preparation of disulfonated bis[4-(3 aminophenoxy)phenyl]sulfone, a disulfonated diamine monomer.
- It is used as a base for the synthesis of high molecular weight homopolymers and copolymers derived from various bisphenols. As a base for the Suzuki coupling of aryl halides with aryl boronic acids. It is used as a reagent in the synthesis of polysubstituted iodobenzene derivatives.
- In the food industry, potassium carbonate is used as a food additive, particularly in the production of cocoa powder and chocolate. It acts as an alkalizing agent and can adjust the pH of certain food products.
- Potassium carbonate anhydrous ACS is used in the preparation of buffer solutions, helping to maintain a stable pH in various chemical and biological processes.
- Potassium carbonate anhydrous ACS is used in photography, glass manufacturing and fire extinguisher powder.
Benefits :
- Meet the toughest regulatory standards for quality and purity
- Highly soluble in water and versatile product
- It contributes to the buffering capacity of solutions, making it valuable in maintaining a stable pH environment
- Safe, effective and affordable product






Reviews
There are no reviews yet.