Buy Ferrous Ammonium Sulfate Hexahydrate ACS (7783-85-9) MF: (NH4)2Fe(SO4)2•6H2O | MW: 392.14. Get High Quality Ferrous Ammonium Sulfate Hexahydrate ACS (7783-85-9) from ChemieR.
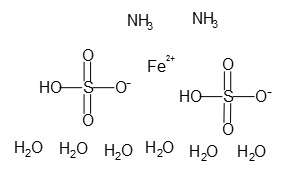
Ferrous Ammonium sulfate Hexahydrate ACS is light green to blue crystalline powder and an inorganic compound. Its chemical formula is Fe(NH4)2(SO4)2 · 6H2O. Ferrous Ammonium sulfate Hexahydrate ACS is known for its exceptional purity and versatility, making it suitable for a wide range of applications. Some alternate names for Ferrous Ammonium sulfate Hexahydrate ACS include Ammonium ferrous sulfate hexahydrate, Ammonium iron(II) sulfate, and Mohr’s salt. Ferrous Ammonium sulfate Hexahydrate ACS readily dissolves in water but is insoluble in ethanol and acetone. It has a melting point of around 100 °C, at which it starts to decompose. Ferrous Ammonium sulfate Hexahydrate ACS is a double salt of iron sulfate and ammonium sulfate and is manufactured by dissolving equimolar amounts of iron(II) sulfate and ammonium sulfate in water, followed by the crystallization of the product.
ChemieR offers a variety of high purity salts and solutions of alkali metals, alkaline earth compounds, perchlorates, heavy metals, rare earths, transition metals, silver/precious metals, and triflates. ChemieR covers wide range of inorganic chemicals, specializing in low moisture and low trace metal grade materials.
ChemieR is innovative and unique product range in laboratory chemicals which includes organic reagents, inorganic reagents, and solvents. Order from our ChemieR brand to meet your budget whilst not compromising on the quality.
In-addition ChemieR also distributes in numerous Laboratory Supplies, Chemicals, Equipment, Instruments, Reagents, Standard Solutions, Buffers, Biological Stains & Indicators and many more, for more information please browse our website (https://chemiereagents.com) or email us sales@chemiereagents.com we will be happy to help you. All ChemieR Products are exclusively distributed by Dawn Scientific Inc (https://dawnscientific.com)
Application :
- Ferrous ammonium sulfate hexahydrate ACS is mainly used in analytical chemistry in redox titrations.
- It is widely used as a source of Fe2+ ions because of its high solubility in water and because it oxidizes much more slowly in air than other ferrous salts like iron(II) sulfate.
- It is also used as a source of Fe2+ to synthesize iron-containing nanoparticles.
- Ferrous Ammonium sulfate hexahydrate also serves as a precursor in the synthesis of iron(III) oxide-hydroxide (FeOOH) nanosheets and as reagents to dope metal oxide-hydroxide nanosheets with iron.
<
Benefits :
- Acts as an iron ion donor for building Fe-S clusters in vitro
- Readily crystallized, and crystals resist oxidation by air
- Low impurities






Reviews
There are no reviews yet.