Buy High Quality Potassium Ferricyanide ACS (13746-66-2) MF: K3[Fe(CN)6] | MW: 329.24.from ChemieR.
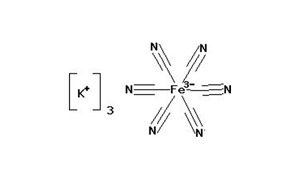
Potassium Ferricyanide ACS is a chemical compound and a bright red salt. It is highly soluble in water and appears as a yellow-green fluorescence. Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. Potassium Ferricyanide ACS is commonly known as potassium hexacyanoferrate(III) or red prussiate. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is Light sensitive. The ACS (American Chemical Society) grade denotes a high level of purity suitable for laboratory and analytical applications.
ChemieR offers a variety of high purity salts and solutions of alkali metals, alkaline earth compounds, perchlorates, heavy metals, rare earths, transition metals, silver/precious metals, and triflates. ChemieR covers wide range of inorganic chemicals, specializing in low moisture and low trace metal grade materials.
ChemieR is innovative and unique product range in laboratory chemicals which includes organic reagents, inorganic reagents, and solvents. Order from our ChemieR brand to meet your budget whilst not compromising on the quality.
In-addition ChemieR also distributes in numerous Laboratory Supplies, Chemicals, Equipment, Instruments, Reagents, Standard Solutions, Buffers, Biological Stains & Indicators and many more, for more information please browse our website (https://chemiereagents.com) or email us [email protected] we will be happy to help you. All ChemieR Products are exclusively distributed by Dawn Scientific Inc (https://dawnscientific.com)
Application :
- Potassium ferricyanide ACS is a potassium salt widely employed as an external indicator in potassium dichromate titrations.
- Addition of potassium ferricyanide in the catholyte can improve the generation of power in microbial fuel cells (MFCs).
- It can be used as a mild reducing agent in certain chemical reactions.
Benefits :
- Meet the toughest regulatory standards for quality and purity
- Used in oxidation-reduction reactions due to its ability to donate electrons
- Safe and easy handling





Reviews
There are no reviews yet.