Buy High Quality Sodium Nitrite ACS (7632-00-0) MF: NaNO2 | MW: 69.00.from ChemieR.
Sodium nitrite ACS appears as a yellowish white crystalline solid. Sodium nitrite is an inorganic sodium salt having nitrite as the counterion. The chemical formula of sodium nitrite is NaNO2. It is soluble in water,methanol,ethanol,ammonia and nitric acid. Slightly soluble diethyl ether. Sodium nitrite (NaNO2) is an inorganic compound that is commonly used as a reagent and catalyst in synthetic organic chemistry. Sodium nitrite (NaNO2), particularly in ACS (American Chemical Society) grade, is a chemical compound primarily used in various industrial and laboratory applications due to its unique properties.
ChemieR offers a variety of high purity salts and solutions of alkali metals, alkaline earth compounds, perchlorates, heavy metals, rare earths, transition metals, silver/precious metals, and triflates. ChemieR covers wide range of inorganic chemicals, specializing in low moisture and low trace metal grade materials.
ChemieR is innovative and unique product range in laboratory chemicals which includes organic reagents, inorganic reagents, and solvents. Order from our ChemieR brand to meet your budget whilst not compromising on the quality.
In-addition ChemieR also distributes in numerous Laboratory Supplies, Chemicals, Equipment, Instruments, Reagents, Standard Solutions, Buffers, Biological Stains & Indicators and many more, for more information please browse our website (https://chemiereagents.com) or email us [email protected] we will be happy to help you. All ChemieR Products are exclusively distributed by Dawn Scientific Inc (https://dawnscientific.com)
Application :
- Sodium nitrite ACS is used as co-catalyst for catalytic oxidation of alcohols under aerobic, solvent-free conditions.
- It is used as a reagent in analytical chemistry, as an electrolyte in electrochemical grinding, as a cooling solution in closed loop systems and as an additive in industrial greases.
- It can be used as a reagent in The Sandmeyer reaction for converting amines into diazo derivatives, and nitration reaction, The oxidative C-C bond formation reaction in the presence of oxygen as the terminal oxidant and The oxidative carbonitration of alkenes in the presence of K2S2O8.
- It acts as a precursor to diazo dyes, nitroso compounds and various organic compounds like pharmaceutical labs.
Benefits :
- Meet the toughest regulatory standards for quality and purity
- As a nitrating agent in organic synthesis
- Versatile and stable product
- Cost effective




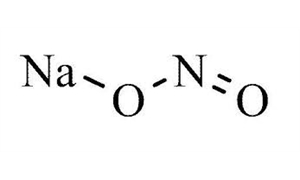

Reviews
There are no reviews yet.