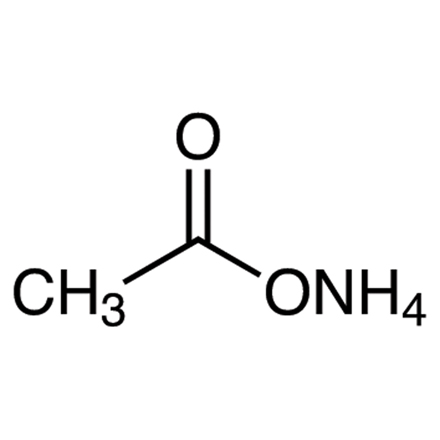
Ammonium Acetate ACS Grade CAS: 631-61-8 | M.F: CH3CO2NH4 | MW: 77.08, Buy now for Reproducible and reliable results from ChemieR Inorganic Reagent. All ChemieR Products are exclusively distributed by Dawn Scientific Inc.
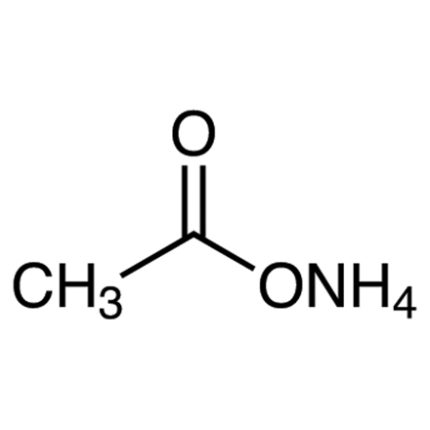
Ammonium acetate ACS Grade is a colorless hygroscopic solid substance. Ammonium acetate is a water-soluble ammonium salt. Its chemical formula is NH4CH3CO2 or NH4OAc. Ammonium acetate or C2H7NO2 appears in the form of a crystalline white solid with a slight acetous odor. It is soluble in water, alcohol, acetone etc. It is an inorganic salt which is derived from the reaction of ammonia and acetic acid. Ammonium acetate ACS grade contains high purity so it is used in many laboratory and industrial applications.
ChemieR provides an extensive selection of high-purity salts, acids, base and high purity solvents, including alkali metals, alkaline earth compounds, perchlorates, heavy metals, rare earths, transition metals, silver and precious metals, and triflates. ChemieR specialize in inorganic chemicals, offering materials with low moisture content and minimal trace metals. ChemieR stands out with a diverse range of laboratory chemicals, from organic and inorganic reagents to solvents.
Application :
- Ammonium acetate ACS is used as a reactant as well as a catalyst in the synthesis of 1,3-oxazine derivatives by three-component condensation reaction with 2-naphthol and aromatic aldehydes.
- It is used as a catalyst as well as a source of ammonia in organic synthesis.
- The compound serves as one of the best sources of ammonia is the Borch reaction during organic synthesis.
- It is also used in the synthesis of symmetrical terpyridine derivatives by treating with aromatic aldehydes and ethyl cyanoacetate.
- It can also be used as a nitrogen source to synthesize benzoxazole derivatives via multicomponent condensation reaction with catechols, and various aldehydes in the presence of Fe(III)−salen complex as a catalyst.
Benefits :
- Meets the toughest regulatory standards for quality and purity
- Eco-friendly and inexpensive compound
- Works best when used as a diuretic
- Hygroscopic inorganic salt








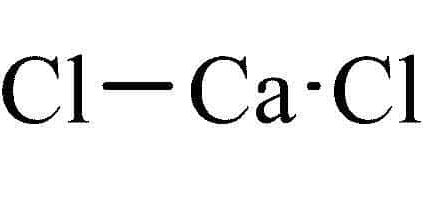



Reviews
There are no reviews yet.