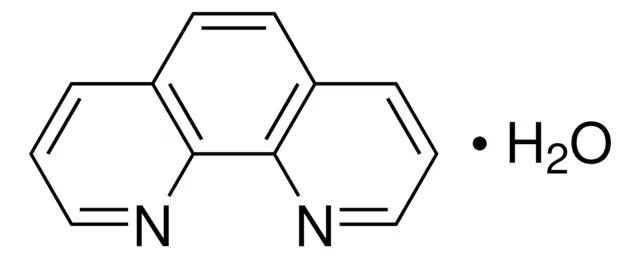
Buy 1,10 Phenanthroline Monohydrate ACS (5144-89-8) MF: C12H8N2-H2O | MW: 198.22. Get High Quality 1,10 Phenanthroline Monohydrate ACS (5144-89-8) from ChemieR.
1,10- Phenanthroline Monohydrate ACS is a colorless crystalline powder with a faint smell. It dissolves easily in both water and organic solvents. The chemical formula for this compound is C12H8N2.H2O. This substance is commonly utilized in the fields of biochemistry and analytical chemistry because of its capacity to create complexes with metal ions. One of its notable complexes is with Fe(II) ion, forming ferroin, which is employed as an indicator in Fe(II) salt titrations. Due to its high purity and quality, ACS grade 1,10-Phenanthroline monohydrate is a valuable reagent in various research and chemical laboratories.
ChemieR offers a variety of high purity salts and solutions of alkali metals, alkaline earth compounds, perchlorates, heavy metals, rare earths, transition metals, silver/precious metals, and triflates. ChemieR covers wide range of inorganic chemicals, specializing in low moisture and low trace metal grade materials.
ChemieR is innovative and unique product range in laboratory chemicals which includes organic reagents, inorganic reagents, and solvents. Order from our ChemieR brand to meet your budget whilst not compromising on the quality.
In-addition ChemieR also distributes in numerous Laboratory Supplies, Chemicals, Equipment, Instruments, Reagents, Standard Solutions, Buffers, Biological Stains & Indicators and many more, for more information please browse our website (https://chemiereagents.com) or email us [email protected] we will be happy to help you. All ChemieR Products are exclusively distributed by Dawn Scientific Inc (https://dawnscientific.com)
Application :
- 1,10 Phenanthroline Monohydrate ACS serves as a redox indicator in for titrations.
- 1,10-phenanthroline monohydrate is a chelator with high affinity for divalent metal ions. It is a widely employed chelate in supramolecular chemistry.
- It is also used in the determination of other metals, such as nickel, ruthenium, and silver.
- When complexed with copper, it possesses nuclease activity that has been used to study DNA-protein interactions.
Benefits :
- High purity compound and meet all the requirements as per ACS specification
- Best redox indicator
- Soluble in Water and organic solvents
- Gives accurate results in titrations
- Forms complexes with metal ions





Reviews
There are no reviews yet.